Quarantine Service
-

Food Inspection
Food products that are imported from other countries can be imported only after being quarantined by the Food and Fisheries Quarantine Service. The purpose of food quarantine is to inspect diseases that are harmful to humans, to prevent pests from spreading across national borders, to prevent the disease from being exported, and to prevent the disease from being transmitted, and to prevent the disease from being attached. Food quarantine is strictly enforced according to the Food Sanitary Law, and Korean government is subject to scientific and rigorous quarantine by international standards.
Products applied by Food Sanitary Law
In case of importing food products, Importer must include the presence of toxic or harmful substances under Article 4, Article 5, Article 6, Article 7, Article 8 and Article 9 of the Food Sanitary Law.
Important Reviews
Import prohibited item status
Proper usage of raw materials (Status for proper food materials or Restricted food materials)
Compliance with the standards for usage of food additives
Compliance with the manufacturing conditions
* Check the amount of solvent and residue used during manufacturing and processing of the food additives.Checking exaggerated advertisement, a false mark, or a big-paved package according to the article 10 of the Food Sanitary Law (Labeling Compliance) and Article 8 of the Enforcement Rules
Standards and specifications of food additive products
Compliance for name of all materials contacted with food in case of package and container packaging
Compliance for the gene recombination food
Submit ' Organic Certificate ' from the International Organic Federation (IFOAM) or organic foods or from the respective Export Administration of exporting countries.
Healthy Food
In case of importing healthy foods, It is considerably required to review all requirements to be complied to the various regulations set forth in the Healthy Food Law before importing them.
Important Reviews
Percentage of ingredients mixed in the product (whether or not the raw materials available as food materials were used, whether or not unacceptable food additives were used, and if the food additive was suitable for use according to the standard of use.)
Manual of the manufacturing process [It is recommended to specify manufacturing conditions in detail during the warming process (Between 00 ℃ and 00 min. for the classification of sterilization, etc.)]
Usage and How to Use
Products and Product Labeling (Status of exaggerated mark or false mark)
Other reference materials (analysis certificate or test certificate, product manual)
* For more information, contact the nearest regional food and Drug Dept (Seoul, Busan, Gyeongin, Daejeon, Daegu, Gwangju).Imported Food Inspection Procedure

Additional Information
- 01Applicant
- - Applicant to import food and health food
- 02Submission Import declaration
- - Report to the Director General of the Local Food and Drug Dept. (Import declaration import in advance from five days before the expected arrival date of importing foods)
- 03Paper Inspection
- - Reference to the inspection to determine the propriety of a report
- 04Sensory Inspection
- - Inspection of the characteristics, status, taste, smell, mark, package status, and prior history of precision inspection of the product to determine compliance with the standards set by Food and Drug Dept.
- 05Precision Inspection
- - inspection performed according to physical, chemical or microbial methods
- 06Random Sample Inspection
- - Inspection carried out in physical, chemical or microbial manner according to the sampling plan as set by Food Safety and Drug Dept
- 07On-site inspection
-
- Conducted in the field of storage warehouse according to the sensory evaluation technique
- Take samples according to the method of collecting and handling samples that are in the food revolution and the health function food revolution
- 08Precision Inspection
- - Inspection according to food standards, food additive standards, health function food component specification, testing method, etc.
- 09Judgment
- - Conduct under the regulations of Food Sanitary Law and the Healthy Food Law
- 10Conformity
- - Conduct under the regulations of Food Sanitary Law and the Healthy Food Law
- 11Issue of Import Permission
- - According to Appendix No. 4 of the Enforcement Regulations of the Food Sanitary Law and the Enforcement Rule of the Health Functional Food Act Annex 20
- 12Import Customs clearance
- - Imported goods after customs duty payment
- 13Domestic Distribution
- - Manufacture, process, and sell of Imported foods
- 14Domestic distribution post management
- - Controlled by Domestic Food and Drug Dept
- 15Nonconformities
- - Not suitable for food under the Food Sanitary Law and the Food Regulations for Health Functions
- 16Non-compliant notification given to importer and Director of the Customs
- 17Returns and Discards
- - Manipulated by Food Sanitary Law, Section 14, Enforcement Regulations, and Enforcement Regulations of Health Function Food Act, Article 10, Paragraph 4
-

Plant Quarantine
Plants imported from a foreign country can only be imported through the quarantine inspection by the Animal and Plant Quarantine Agency. The purpose of plant quarantine is to prevent diseases and pests from spreading across the borders and to prevent the disease and pests from being transmitted. Plant quarantine procedures are carried out under the Plant Quarantine Law, and Korea operates the following scientific standards by revising the Plant Prevention Act to comply with the WTO / SPS Convention
What is a regulatory disease?
If not taken any measures, such as disinfection or disposal during the disease, It may be considered harmful to plants and divided into restricted quarantine of disease and insect pest (prohibited and managed) and non-restricted quarantine of disease and insect pest.
Quarantine of disease and insect pest
It doesn’t exist in the country but may cause serious damage when imported to the country and distributed in some parts of the country. It is It is divided into prohibited and managed disease which measures are being taken to prevent outbreaks and other possible damage.
Prohibited disease and insect pest
it is admitted to cause serious damage to plants If the plant is not discarded or returned to origin which disease and insect pest are prohibited from importing as regulated by Animal and Plant Quarantine Agency.
Managed disease and insect pest
The disease and insect pest, when imported, may cause damage to other plants is significant in in recognition by agriculture and forestry quarantine agency.
Non-restricted quarantine of disease and insect pest.
The disease and insect pest which cause damage so that cannot be economically accommodated on the all edible plants.
Health food
In case importing health function foods, Importer must review overall regulations set by respective laws for the foods before import.
What is provisionally restricted disease and insect pest?
The disease and insect pest discovered during quarantine as first time or in the process of analyzing the risk of disease and insect pest required to take measures such as disinfection and disposal tentatively.
Non-restricted disease and insect pest of quarantine?
The disease and insect pest is widely distributed in Korea and is attached to imported agricultural products, but does not take quarantine measures such as disinfection.
Quarantine procedures of Plant import

-

Livestock / Animal quarantine
Livestock / animal quarantine is allowed only after the quarantine of the Animal and Plant Quarantine Agency. The purpose of quarantine inspection on livestock products / animals is to prevent the spread or introduction of diseases related to livestock infectious diseases, such as foot-and-mouth disease, mad cow disease and avian influenza, and to prevent the quarantine of livestock products and animals in Korea.
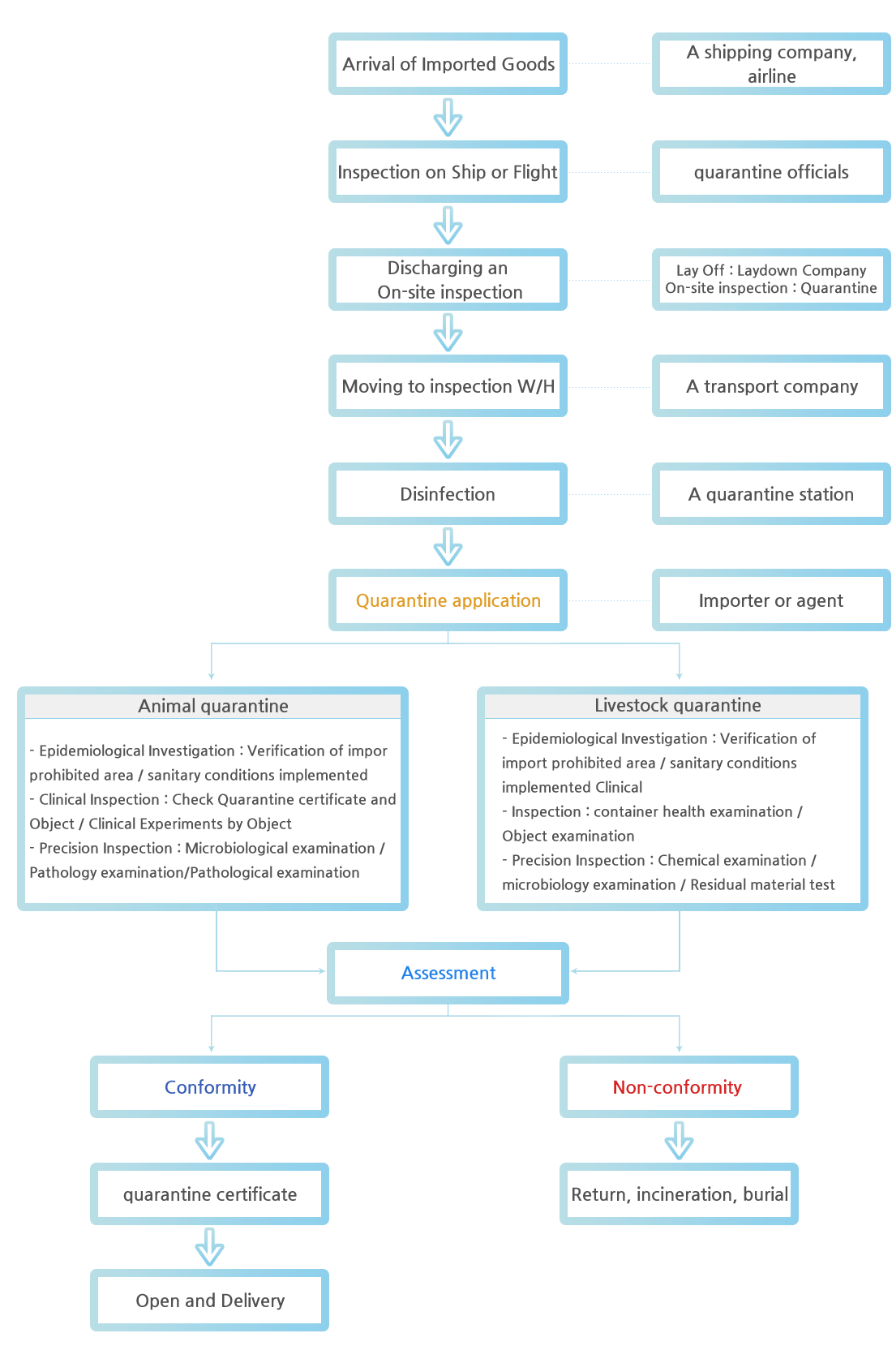
Quarantine procedures of imported Animal and livestock
Additional Information
quarantine of the imported animals
- 01Submit an animal declaration to the Reginal director Animal and Plant Quarantine Agency before importing according to the relevant regulations
- 02Reporting imported arriva
- - Importer required to declare by phone or document including the place of arrival, the person who imports the animal, and the plan for unloading and transport and the place of arrival.
- 03Inspection on Ship or Aircraft
-
-Inspection of dedicated ships is carried out at outer of ports.
- Inspection of dedicated aircraft should be carried out in a reasonable location for livestock quarantine.
- Inspection items
·Inspection on ships in case of import forbidden countries is carried out at outer of ports.
·Check written details of quarantine certificates issued by exporter
·Survey on the conformity of Sanitary conditions regulated by Korea
- 04Unloading and transportation
- - Transportation is safely carried out under control by prior quarantine regulations of quarantine inspector and is subject to prior instructions from the quarantine officer (unload company, transport company)
- 05Mooring at quarantine station
- - mooring during quarantine period
- 06Quarantine Application
-
- Submission of document
·Quarantine Application
·Quarantine certificate of export country (statement of relevant regulations or the sanitary conditions agreed with importing country)
·Reference documents (B/L, invoice, etc.)
- 07Epidemiological Investigation
-
- Examination of quarantine application forms and attachments
- Check examination items of Ship or Aircraft conditions
- Other necessary factors required for epidemiological examination
- 08Clinical inspection and precision inspection
-
- performed daily on each animal object according to the guidelines for livestock diseases
- Conduct a precision inspection according to the infection examination guidance for each animal.·Microbiology, pathology, and serum examination
- 09Assessment
-
- Conformity : Issue of quarantine certificates
- Non-conformity: Return, incineration or burial
-

Fishery product quarantine
Fish products that are imported from other countries can be imported only after being quarantined by the Animal and Plant Quarantine Agency. The purpose of marine products quarantine is to prevent diseases and harmful insects that damage fish products from spreading across the borders, to prevent the disease and its attachment.
Additional Information
- 01Check Import Manifest
- - Shipping Carrier or Air Carrier or their agents submit import manifest in EDI or Paper documents
- 02Inspection on Ship or Aircraft
- 03Unloading and Transportation (Quarantine notification)
- - Transport to quarantine stations is carried out in a safe manner under quarantine after prior instructions from the quarantine officer
- 04Receiving at quarantine station
- - Container opening status, Sensory inspection, On-site inspection.
- 05Quarantine application
-
- Submission relevant documents
·Inspection application form, B/L, and Quarantine Certificate of Exporter and other reference documents
- 06Epidemiological Investigation
- - Check status for import prohibited areas and passing through of import prohibited areas
- 07Precision Inspection (inspection if necessary)
- - Microbiology, Chemical and Residual material Inspection
- 08Assessment
-
- Conformity: Issue quarantine certificate
- Non-conformity: Return, Incineration or Burial
Fishery product quarantine
Legal grounds
- Article 88 of the Agricultural and Marine Products Quality Management Law (Inspection on Fishery Products) and Article 96 (Re-examination)
Products for Quarantine
- Fish products and fisheries processing products that are announced by the Minister of Agriculture, Forestry and Fisheries as in case an inspection is required by contract with foreign country or upon the request of an export country.
Inspection under a foreign contract
United States : Fresh and frozen fishes and shells
EU Countries : Live Shells, Hyperphycetes and Marine Reproduction (fishery and cultured fishery products)
China : Raw Fishery materials, products, Simple reprocessed products (Processed fishery products that is cut, heated, dried, salted or salted to a point where the prototype can be recognized without using any additives or other materials other than edible salt)
Japan : Fresh oyster, Processed puffer fish, and Flatfish for export
Vietnam : Raw Fishery materials, Processed fishery products that has been processed, such as cutting, heating, steaming, drying or salting, to the point where it is possible to recognize the prototype without using food additives or other raw materials.
Indonesia : Raw Fishery materials, products, Simple reprocessed products (Processed fishery products that is cut, heated, dried, salted or salted to a point where the prototype can be recognized without using any additives or other materials other than edible salt)
Thai : Raw Fishery materials, products, Simple reprocessed products (Processed fishery products that is cut, heated, dried, salted or salted to a point where the prototype can be recognized without using any additives or other materials other than edible salt).
* Only fisheries products and processed fisheries products in factories registered as production and processing facilities that meet sanitary standards (including ships for EU products)
* Fishery products are marked with the registration number of the country's name, manufacturing, and processing facilities.
* Legal grounds : Notification on registration of production and processing facilities
- Inspection by standards and standards requested by the applicant or importer
- Inspection with attached documents that specify the standards and specifications and inspection exemption on specific items
Inspection standards
- Standards of inspection of fisheries products and fisheries products (Notice No. 2012-134 of the Office of Agriculture, Forestry and Fisheries Inspection)
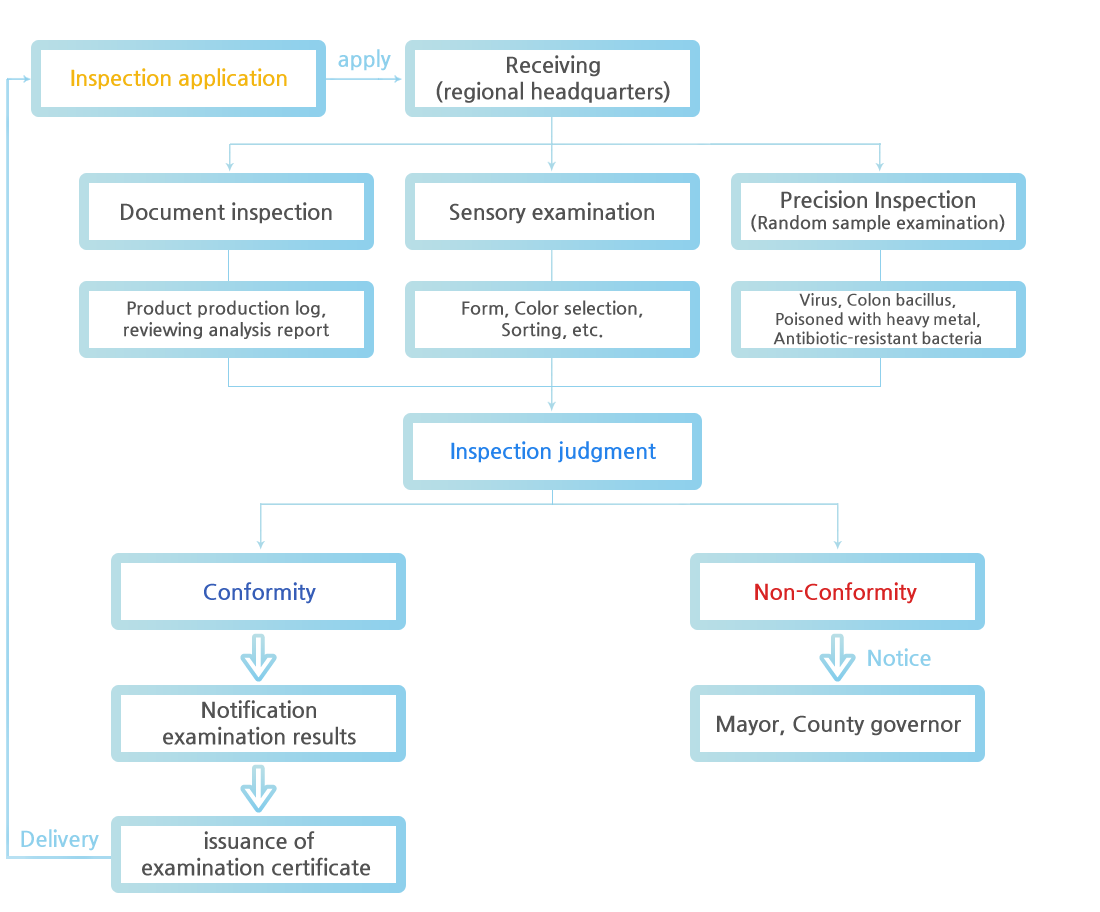
Inspection procedure
Inspection means
※ Document Inspection
Inspection to determine its suitability by reviewing the application documents
Examine the production status at designated sea area (Limited to fisheries products and processed fisheries products that must be produced in designated sea area)
Check if a production and processing facility is registered, and if it complies with the facility sanitary standards and conformance to standards of risk element management criteria.
Permit status of deep-sea fishing industry under Article 41 para 1 of the Fisheries Business Act, etc.
※ Sensory Inspection
Inspection to determine the suitability (proper or incorrect) of the five senses and Inspection to be done for fishery products and processed fishery products that require confirmation of quality standards, package conditions, marks, and specifications
Fishery and processed fishery products for which the applicant requests sanitary certificate.
※ Precision Inspection
Inspection to determine the propriety of a physical, chemical, or microbial method.
Fishery and processed fishery products for which the applicant or foreign standards for inspection demand require an analysis certificate.
Fishery and processed fishery products that are recognized to require precision inspection as a result of a sensory examination.
Fishery that is produced and processed at the production and processing facilities of fisheries products and fisheries products if hazardous materials are detected in the fisheries products and fisheries products exported according to the foreign standards.
 KOREAN
KOREAN ENGLISH
ENGLISH


